13.1 Classify the following amines as primary, secondary or tertiary.
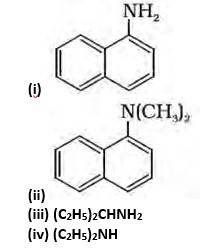
13.1 Classify the following amines as primary, secondary or tertiary.

-
1 Answer
-
(i) Primary, because the nitrogen atom is attached to the only 1 carbon atom.
(ii) Tertiary, because the Nitrogen atom is attached to the 3 carbon atoms.
(iii) Primary, because the nitrogen atom is attached to the only 1 carbon atom.
(iv) Secondary, because the nitrogen atom is attached to the only 1 carbon.
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
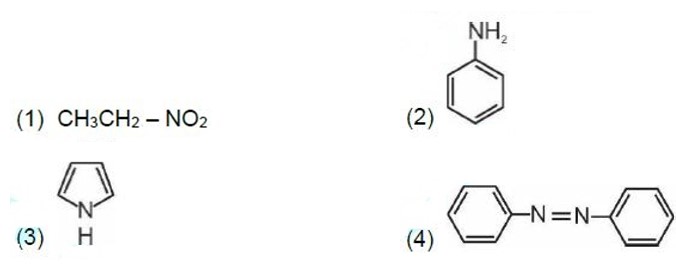
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
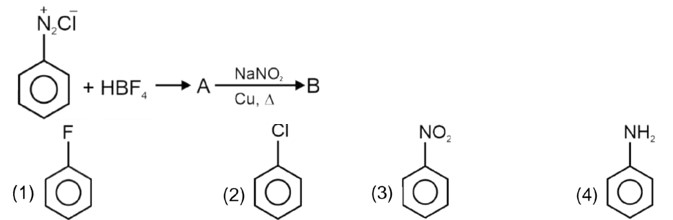
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 65k Colleges
- 1.2k Exams
- 688k Reviews
- 1800k Answers