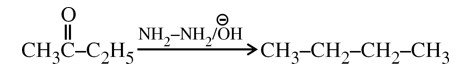
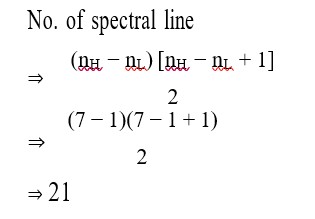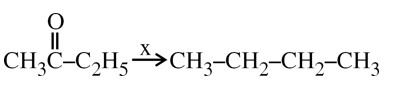In Carius method of estimation of halogen, 0.172 g of an organic compound showed presence of 0.08 g of bromine. Which of these is the correct structure of the compound?
In Carius method of estimation of halogen, 0.172 g of an organic compound showed presence of 0.08 g of bromine. Which of these is the correct structure of the compound?
Option 1 -
a
Option 2 -
b
Option 3 -
c
Option 4 -
d
-
1 Answer
-
Correct Option - 4
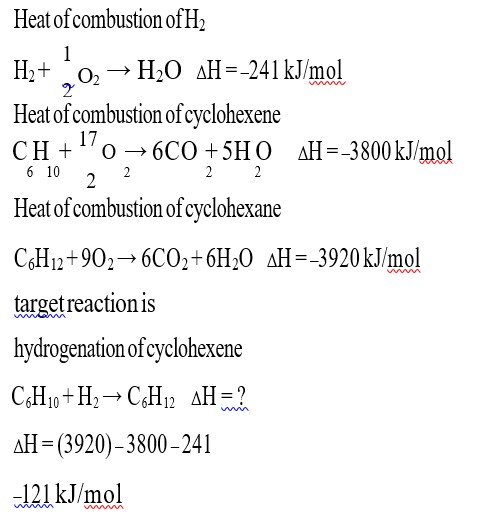
Detailed Solution:Mole of Bromine = 0.08/80 = 10? ³ mole
Molar mass of compound = 0.172/M = 10? ³
M = 0.172/10? ³ = 172g
Molar mass of
Similar Questions for you
Kindly consider the solution
Fact.
No. of spectral line
Wolf Kishner Reduction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers