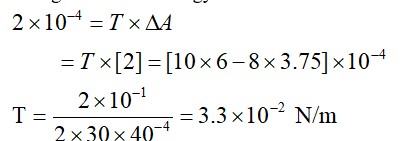12.4 A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom make a transition from the upper level to the lower level?
12.4 A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom make a transition from the upper level to the lower level?
-
1 Answer
-
12.4 Separation of two energy level of atom, E = 2.3 eV = 2.3 J = 3.68
Let be the frequency of radiation emitted when the atom transits from upper level to lower level.
We have the relation for energy as , where
h = Planck's constant = 6.626 Js
Then = Hz = 5.55 Hz
Hence the frequency is 5.55 Hz
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers