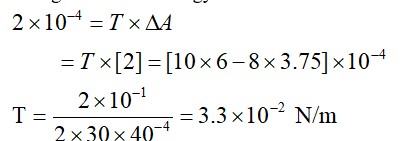12.9 A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. What series of wavelengths will be emitted?
12.9 A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. What series of wavelengths will be emitted?
-
1 Answer
-
12.9 It is given that the energy of the electron beam used to bombard gaseous hydrogen at room temperature is 12.5 eV. Also, the energy of the gaseous hydrogen in its ground state at room temperature is – 13.6 eV.
When gaseous hydrogen is bombarded with an electron beam, the energy of the gaseous hydrogen becomes -13.6 + 12.5 eV i.e. -1.1 eV
Orbital energy is related to orbit level(n) as:
E = eV
For n = 3, E = eV = - 1.5 eV
This energy is approximately equal to the energy of the gaseous hydrogen. It can be concluded that the electron has jumped from n = 1 to n = 3 level.
During the de-excitation, the electron can jump from n=3 to n=1 d
...more
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers