1L aqueous solution of H2SO4 contains 0.02m mol H2SO4. 50% of this solution is dilutd with deionized water to give 1L solution (A). In solution (A), 0.01m mol of H2SO4 are added. Total m mols of H2SO4 in the final solution is ________* 103 m mols
1L aqueous solution of H2SO4 contains 0.02m mol H2SO4. 50% of this solution is dilutd with deionized water to give 1L solution (A). In solution (A), 0.01m mol of H2SO4 are added. Total m mols of H2SO4 in the final solution is ________* 103 m mols
1000 ml solution contains 0.02 milli mole (mm)
500 ml solution contains 0.02 m.m
Solution made 1000 ml with H2O
m.m in final solution = 0.01 mm
Solution (A) + 0.01 m. m H2SO4
= 0.01 + 0.01
= 0.02 m.m
= 0.00002 * 103 mm
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
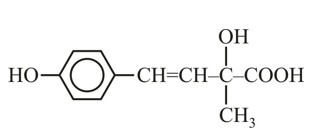
It has chiral centre and differently di substituted double bonded carbon atoms.
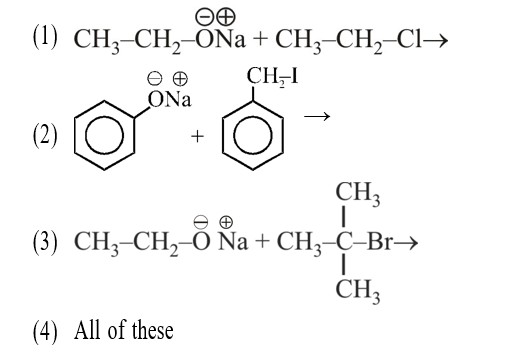
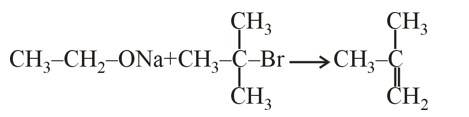
Rate of ESR ∝ No. of α – H (Hyperconjugation)
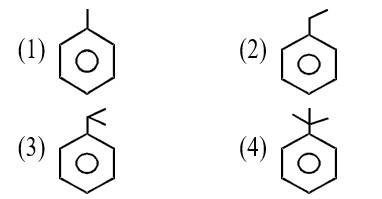
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering