From the following pairs of ions which one is not an iso-electronic pair?
From the following pairs of ions which one is not an iso-electronic pair?
Option 1 - <p>O²⁻, F⁻<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 2 - <p>Na⁺, Mg²⁺<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 3 - <p>Mn²⁺, Fe³⁺<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 4 - <p>Fe²⁺, Mn²⁺</p>
2 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
V
Answered by
5 months ago
Correct Option - 4
Detailed Solution:
Total no. of e?
? Fe → 3d?4s², Fe? ² → 3d? 24
? Mn → 3d?4s², Mn? ² → 3d? 23
Similar Questions for you
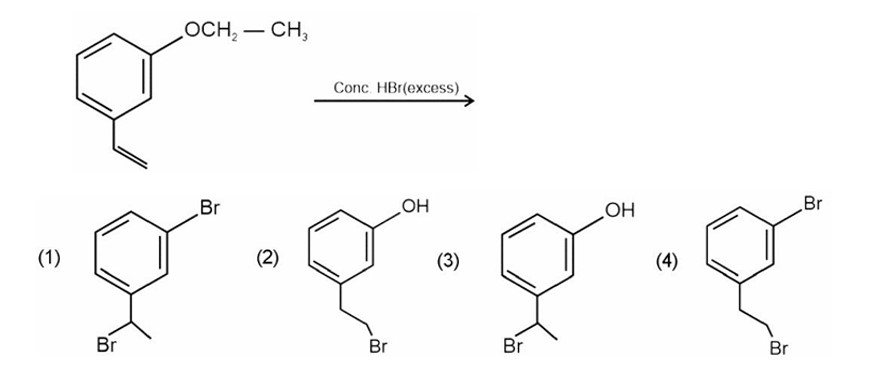
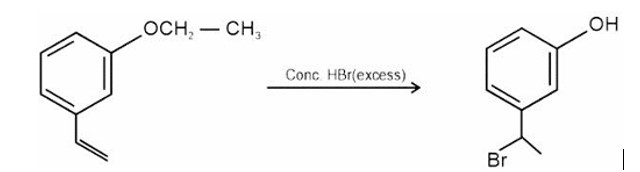
HBr adds to alkene in accordance with Markovnikov's rule.
Delocalisation of
To study Hydrocarbons for NEET, you can use the Hydrocarbons Class 11th NCERT solutions PDF.
Alkanes, Alkenes, Alkynes, and Aromatics hydrocarbons are the four main hydrocarbons.
Hydrocarbons are organic compounds made of only carbon and hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering