Given below are two statements:
Statement I : is square planar and diamagnetic complex, with dsp2 hybridizatin for Ni but is tetrahedral, paramagnetic and with sp3 hybridization for Ni.
Statement II : both have same d-electron configuration have same geometry and are paramagnetic.
In light the above statements, choose the correct answer from the options given below:
Given below are two statements:
Statement I : is square planar and diamagnetic complex, with dsp2 hybridizatin for Ni but is tetrahedral, paramagnetic and with sp3 hybridization for Ni.
Statement II : both have same d-electron configuration have same geometry and are paramagnetic.
In light the above statements, choose the correct answer from the options given below:
CN- is strong field ligand
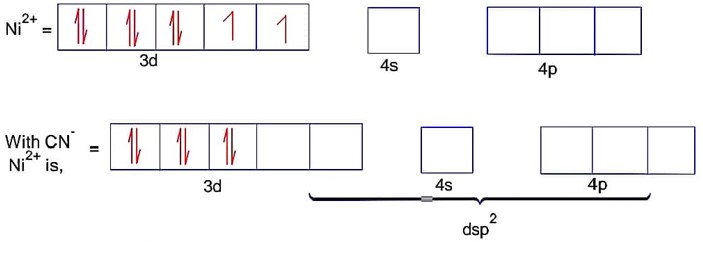
Here; is square planar and diamagnetic.
Ni = 4s23d8 Co is strong field ligand.
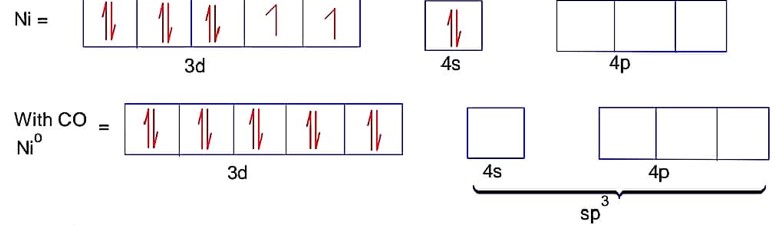
Here ; is tetrahedral and diamagnetic
has 3d8 configuration while has 3d10 configuration.
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
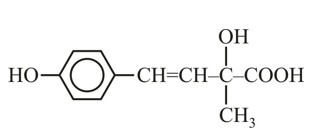
It has chiral centre and differently di substituted double bonded carbon atoms.
Rate of ESR ∝ No. of α – H (Hyperconjugation)
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Coordination Compounds 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering