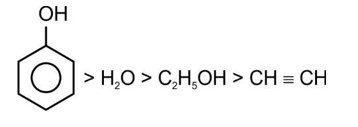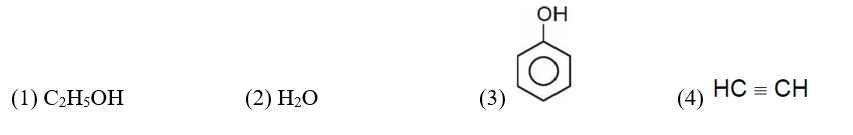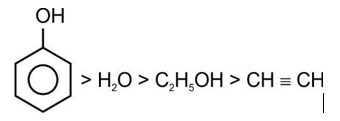In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it?
In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it?
Option 1 - <p>HF < HCl < HBr < HI : Increasing acidic strength</p>
Option 2 - <p>H₂O < H₂S < H₂Se < H₂Te : Increasing pKₐ</p>
Option 3 - <p>NH₃ < PH₃ < AsH₃ < SbH₃ : Increasing acidic character<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 4 - <p>CO₂ < SiO₂ < SnO₂ < PbO₂ : Increasing oxidizing power</p>
3 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
V
Answered by
5 months ago
Correct Option - 2
Detailed Solution:
H? O < H? S < H? Se < H? Te
Down the group acidic strength increases
So pK? value decreases
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering