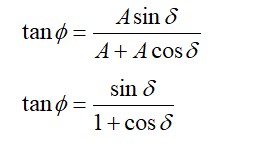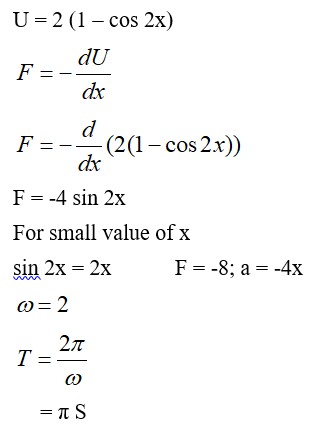At constant temperature an increase in the pressure of a gas by 5% will decrease its volume by
At constant temperature an increase in the pressure of a gas by 5% will decrease its volume by
Option 1 - <p>5%</p>
Option 2 - <p>5.26%</p>
Option 3 - <p>4.26%</p>
Option 4 - <p>4.76%</p>
2 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
R
Answered by
4 months ago
Correct Option - 4
Detailed Solution:
If then of
From Boyle's law,
Fractional change in volume is
Therefore, percentage change in volume is
That is, volume decreases by
Similar Questions for you
From A to B the process is isobaric
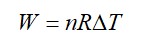
= W = 2 × R (600 - 350)
= 500 R
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

physics ncert exemplar solution class 12th chapter one 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering