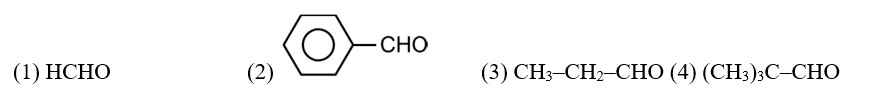
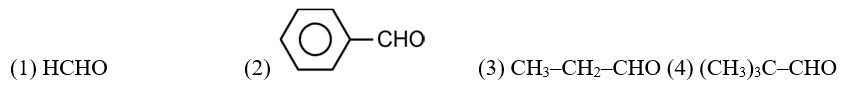
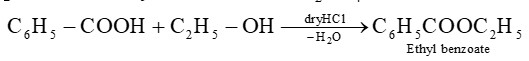Boiling points of aldehydes are higher than hydrocarbons. It is due to
Boiling points of aldehydes are higher than hydrocarbons. It is due to
Option 1 - <p>Weak molecular association</p>
Option 2 - <p>High intermolecular hydrogen bonding</p>
Option 3 - <p> High molecular masses</p>
Option 4 - <p>None of these</p>
1 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Correct Option - 2
Detailed Solution:
Ethyl benzoate can be prepared by heating benzoic acid with ethyl alcohol in presence of dry HC1 or conc. The reaction is called as esterification reaction.
Similar Questions for you
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering