Calculate the molarity of a solution having density = 1.5g/mL %(w/w) of solute is 36% and molecular 36 g/mol
Calculate the molarity of a solution having density = 1.5g/mL %(w/w) of solute is 36% and molecular 36 g/mol
5 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Assume mass of solution
= 100g
Mass of solute = 36gm
Moles of solute = 1
Molarity=
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
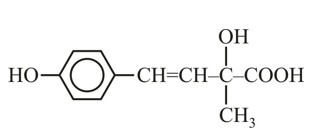
It has chiral centre and differently di substituted double bonded carbon atoms.
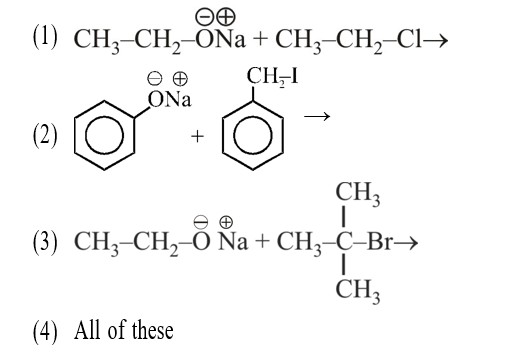
Rate of ESR ∝ No. of α – H (Hyperconjugation)
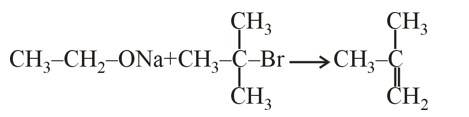
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Solutions 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering