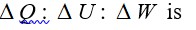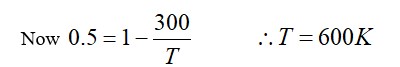The temperature of equals masses of three different liquids x, y and z are 10°C, 20°C and 30°C respectively. The temperature of mixture when x is mixed with y is 16°C and that when y is mixed with z is 26°C. Temperature of mixture when x and z are mixed will be:
The temperature of equals masses of three different liquids x, y and z are 10°C, 20°C and 30°C respectively. The temperature of mixture when x is mixed with y is 16°C and that when y is mixed with z is 26°C. Temperature of mixture when x and z are mixed will be:
Option 1 - <p>8.32°C </p>
Option 2 - <p>23.84°C</p>
Option 3 - <p>25.62°C</p>
Option 4 - <p>20.28°C</p>
2 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
V
Answered by
5 months ago
Correct Option - 3
Detailed Solution:
msx (16 – 10) = msy (20 – 16)
Similar Questions for you
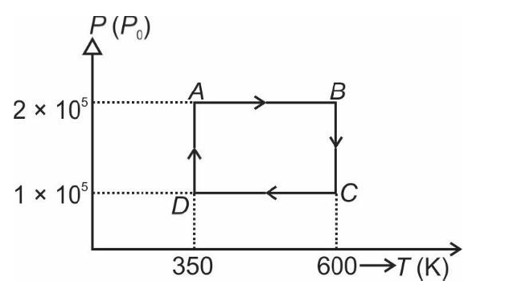
From A to B the process is isobaric
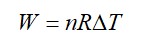
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering