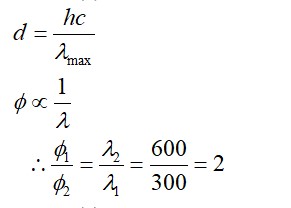10.3. Why are alkali metals not found in nature?
10.3. Why are alkali metals not found in nature?
2 Views|Posted 9 months ago
Asked by Shiksha User
1 Answer
V
Answered by
9 months ago
All the alkali metals have one valence electron, ns1 outside the noble gas core. The loosely held s-electron in the outermost valence shell of these elements makes them the mostelectropositive metals, i.e. they readily lose electron to give monovalent M+ ions. Hence, they are never found in free sta
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering