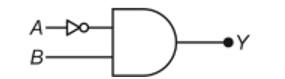
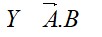11.15. If B-Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
11.15. If B-Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
2 Views|Posted 9 months ago
Asked by Shiksha User
1 Answer
V
Answered by
9 months ago
B-Cl bond has dipole moment because of polarity. In BCl3, since the molecule is symmetrical (planar), the polarities cancel out and hence the dipole moment is zero
Similar Questions for you
From BF3 to BI3 Lewis acidic strength increases
F2 is the strongest oxidising agent
HClO4 is the most acidic compound.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering