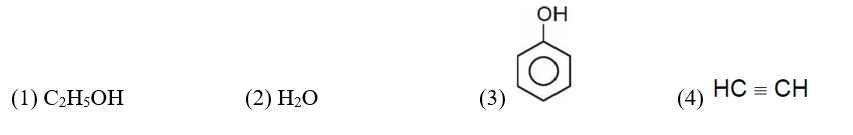11.43
The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para- substituted compound.
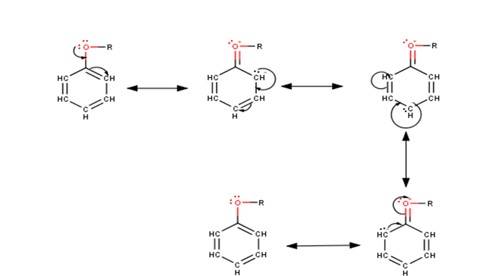
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
The Friedel-Crafts alkylation of anisole gives p-methylanisole as the major product.
The mechanism is given below:
2- The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
Nitration of anisole gives p-nitroanisole as the major product.
The mechanism is given below:
3- The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
Bromination of anisole in ethanoic acid medium gives p-bromoanisole as the major product.
4-The driving force of all the reactions given to the question is that the alkoxy group is an ortho and para directing group because it exerts its +R effect in the benzene ring. Para position being
comparatively more stable than the ortho position is usually preferred because ortho position leads to stearic hindrance, hence the major product is mostly the para-substituted compound.
As seen from the resonating structures above the structure in which the negative charge is in the para position will form a more stable product when attacked by an electrophile. Hence in the following reactions, we will be considering that resonating structure as the starting material.
The Friedel-Craft's acetylation of anisole gives 4- methoxyacetophenone as the major product
The mechanism is given below: