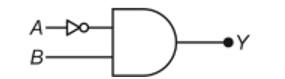
11.8. Write reactions to justify amphoteric nature of aluminium.
11.8. Write reactions to justify amphoteric nature of aluminium.
0 Views|Posted 9 months ago
Asked by Shiksha User
1 Answer
V
Answered by
9 months ago
Aluminium reacts with acid as well as base. This shows amphoteric nature of aluminium.
2Al (s) + 6HCl (dil.) →2AlCl3 (aq) + 3H2 (g)
2Al (s) + 2NaOH (aq) + 6H2O (l) →2Na+ [Al (OH)4]– (aq) + 3H2 (g)
Similar Questions for you
From BF3 to BI3 Lewis acidic strength increases
F2 is the strongest oxidising agent
HClO4 is the most acidic compound.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering