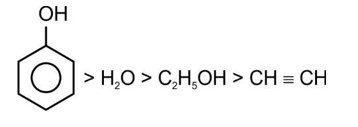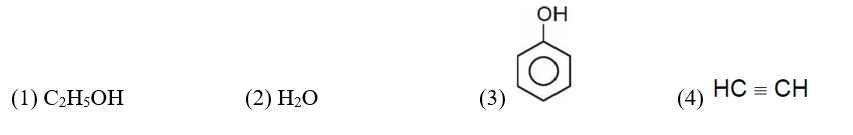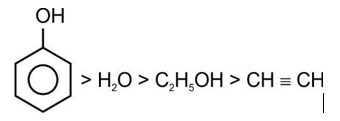11.80 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
11.80 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
-
1 Answer
-
11.80
The preparation of ether by acid dehydration of primary alcohol involves the nucleophilic addition of alcohol molecule to the protonated alcohol molecule as shown below: -
However, under these conditions secondary and tertiary alcohols forms alkenes rather than ethers. The reason for this being that due to stearic hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not take place. Instead protonated 20 and 30 alcohols lose a molecule of water to form stable carbocations. The stable carbocations so formed prefers to lose a proton to form alkenes instead of forming ethers by undergoing
...more
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers