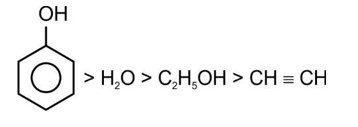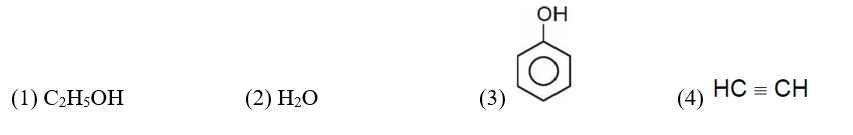11.86 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place: Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
11.86 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place: Give a mechanism for this reaction. (Hint : The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.
11.86

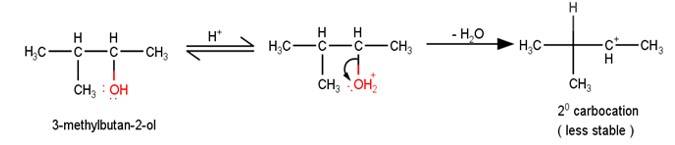
The first step in the mechanism of th e given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
e given reaction is protonation of the alcohol followed by loss of water to give a 20 carbocation.
2. The next step is a rearrangement of the 20 carbocations formed in the above step is less stable it rearranges by a 1,2-hydride shift to form more stable 3°
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering