13.3 Account for the following:
(i) pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is o– and p– directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
13.3 Account for the following:
(i) pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is o– and p– directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
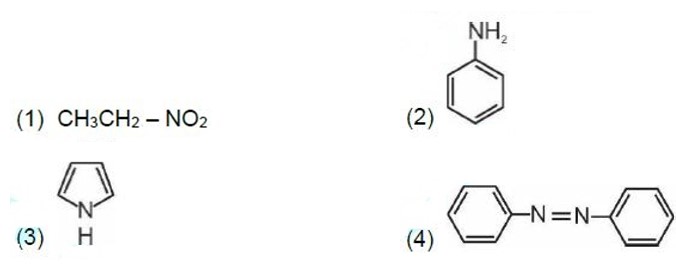
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
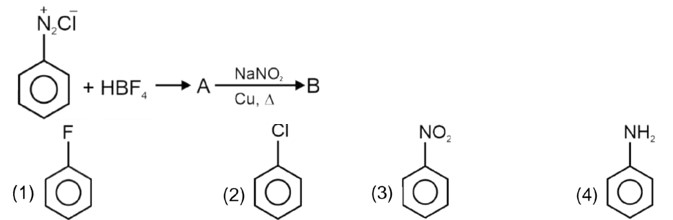
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering