13.3 How will you convert
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
(iii) Cl–(CH2)4–Cl into hexan-1,6-diamine?
13.3 How will you convert
(i) Benzene into aniline
(ii) Benzene into N, N-dimethylaniline
(iii) Cl–(CH2)4–Cl into hexan-1,6-diamine?
Benzene into aniline
When Benzene is treated with HNO3/H2SO4 it forms nitrobenzene. When Nitrobenzene reduced with Sn/HCL it forms Aniline because Sn/HCl is a reducing Mixture.
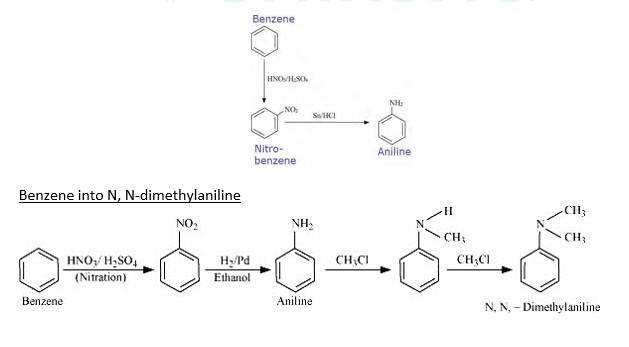
When Benzene is reacted with nitrating mixture it forms nitrobenzene. When it Reduced H2/Pd in ethanol or Sn/HCl, it forms An
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
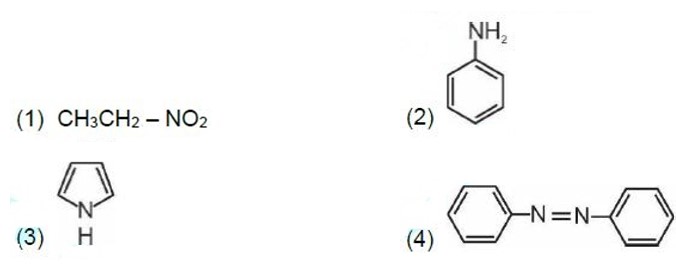
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
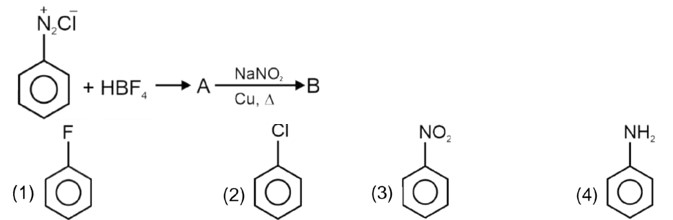
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering