13.6 Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
13.6 Describe a method for the identification of primary, secondary and tertiary amines. Also write chemical equations of the reactions involved.
Primary amine: A primary (1°) amine is an amine that has the following general structural formula. R= alkyl or aryl group Secondary amine: A secondary (2°) amine is an amine that has the following general structural formula. R1 and R2= alkyl or aryl group Tertiary amine: A tertiary amine is an amine that has the following structure R1, R2 and R3 are alkyl or aryl groups Identification of Primary, Secondary and Tertiary amines Primary, secondary and tertiary amines can be identified by the following test: Hinsberg's test: This is an excellent test for the identification of primary, secondary and tertiary amines. In this test, the amine is shaken with benzenesulphonyl chloride ( Hinsberg's reagent) in the presence of an excess of aqueous KOH solution when (i) A primary amine gives a clear solution which on acidification gives an N-alkylbenzene sulphonamide which is soluble in Due to the presence of strong electron withdrawing sulphonyl group in the sulphonamide, the H-atom attached to nitrogen can be easily released as a proton. So it is acidic and dissolves in alkali. (ii) A secondary amine reacts with Hinsberg's reagent to give a sulphonamide which is soluble in There is no H-atom attached to the N-atom in the sulphonamide Therefore it is not acidic and soluble in alkali. (iii) A Tertiary amine does not react with Hinsberg's reagent at all |
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
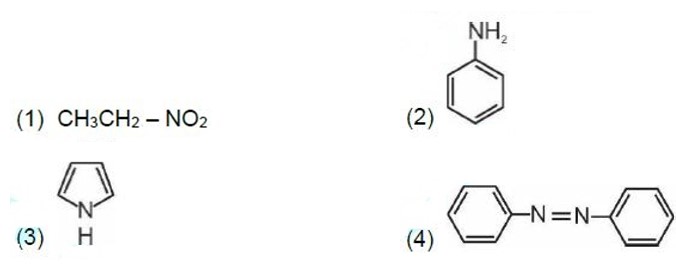
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
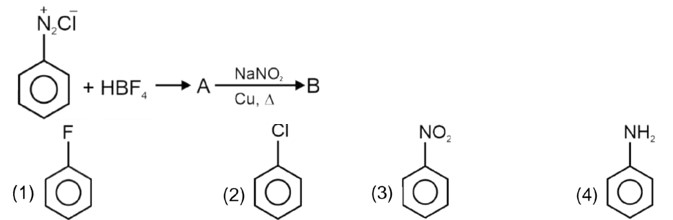
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering