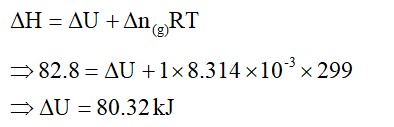2.2g of nitrous oxide (N2O) gas is cooled at a constant pressure of 1 atm from 310 K to 270 K causing the compression of the gas from 217.1mL to 167.75mL. The change in thermal energy of the process, is '-x' J. The value of 'x' is________. [Nearest Integer]
(Given : atomic mass of N = 14 g mol-1 and of O = 16 g mol-1.
Molar heat capacity of N2O is 100 J K-1 mol-1)
2.2g of nitrous oxide (N2O) gas is cooled at a constant pressure of 1 atm from 310 K to 270 K causing the compression of the gas from 217.1mL to 167.75mL. The change in thermal energy of the process, is '-x' J. The value of 'x' is________. [Nearest Integer]
(Given : atomic mass of N = 14 g mol-1 and of O = 16 g mol-1.
Molar heat capacity of N2O is 100 J K-1 mol-1)
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering