4.24 The experimental data for decomposition of N2O5 [2N2O5 → 4NO2 + O2 ] in gas phase at 318K are given below:
(i) Plot [N2O5 ] against t.
(ii) Find the half-life period for the reaction.
(iii) Draw a graph between log[N2O5 ] and t.
(iv) What is the rate law ?
(v) Calculate the rate constant.
(vi) Calculate the half-life period from k and compare it with (ii).
t/s
0
400
800
1200
1600
2000
2400
2800
3200
102 *
[N2O5]/mol L-1
1.63
1.36
1.14
0.93
0.78
0.64
0.53
0.43
0.35
4.24 The experimental data for decomposition of N2O5 [2N2O5 → 4NO2 + O2 ] in gas phase at 318K are given below:
(i) Plot [N2O5 ] against t.
(ii) Find the half-life period for the reaction.
(iii) Draw a graph between log[N2O5 ] and t.
(iv) What is the rate law ?
(v) Calculate the rate constant.
(vi) Calculate the half-life period from k and compare it with (ii).
|
t/s |
0 |
400 |
800 |
1200 |
1600 |
2000 |
2400 |
2800 |
3200 |
|
102 * [N2O5]/mol L-1 |
1.63 |
1.36 |
1.14 |
0.93 |
0.78 |
0.64 |
0.53 |
0.43 |
0.35 |
4.24
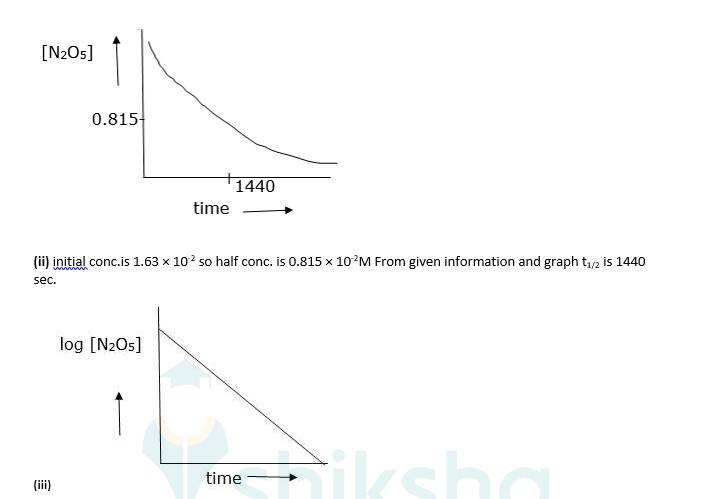
(iv) As log [N2O5] vs time is a straight line given reaction is first Hence its rate law will be, Rate = k [N2O5]
(v) The slope of above graph is slope = 0.000209 K = 303 * slope
⇒4.82 * 10-4sec-1
Now, t1/2 = 0.693/K.
⇒0.693/4.82 * 10-4
⇒t1/2 = 1438 sec. which is almost equal to (ii)
Similar Questions for you
Kindly go through the solution
Ea = 216.164kJ/mol 216
Reaction rate is used to measure how fast or slow reactions occur per unit time. The rate constant is a proportionality factor that remains constant for every reaction.
Yes, in elementary reactions, order and molecularity can be the same, but this is not always the case because order is an experimental quantity, and molecularity is a theoretical concept.
Reaction Kinetics, also known as chemical kinetics, is the study of the rate of chemical reaction and the factors affecting the reaction rate, such as temperature, concentration, and catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering
