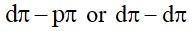6.21 Why copper matte is put in silica lined converter?
6.21 Why copper matte is put in silica lined converter?
-
1 Answer
-
Copper matte contains Cu2S and FeS. When a blast of hot air is passed through molten matte took in a silica lined convertor, FeS present in matte is oxidized to FeO which combines with Silica (SiO2) to form FeSiO3, slag.

When the whole of iron has been removed as slag, some of the Cu2S undergoes oxidation to form Cu2O which then reacts with more Cu2O to form copper metal.

Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
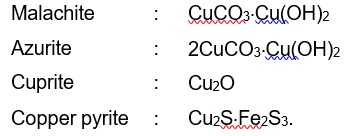
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers