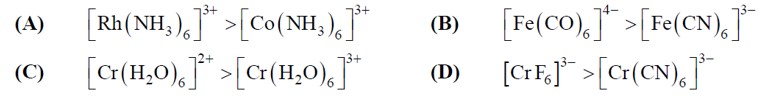9.28 What is crystal field splitting energy? How does the magnitude of ?o decide the actual configuration of d orbitals in a coordination entity?
9.28 What is crystal field splitting energy? How does the magnitude of ?o decide the actual configuration of d orbitals in a coordination entity?
-
1 Answer
-
The difference between the energies of the two set of the d orbitals is called as crystal field splitting energy (CFSE). The degenerate d orbitals split into two levels i.e t2g and eg level due to the presence of the ligands. This splitting of the degenerate orbitals due to the ligand is called as crystal field splitting and the energy difference between the two levels is called as crystal field splitting energy.
After the splitting of the degenerate orbitals has taken place the filling of the electrons takes place. Now first 3 electrons goes into the lower energy three t2g orbitals. The fourth electron can be filled in two ways:
It can
...more
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
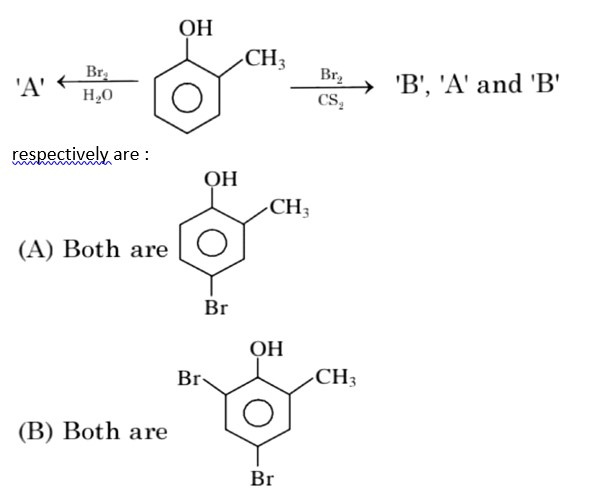
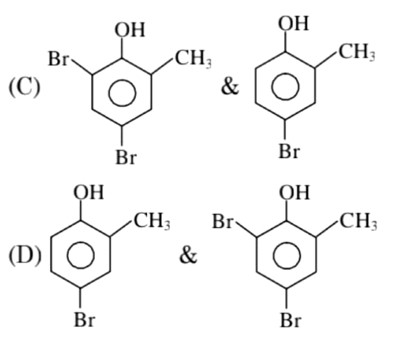
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers