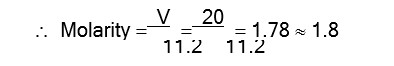9.33. What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15,19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
9.33. What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15,19, 23 and 44 with dihydrogen? Compare their behaviour towards water.
9.33. Atomic number 15 is of phosphorus. The hydride is PH3 and its nature is covalent. Atomic number = 19 is of potassium. The hydride is KH and it is ionic in nature. Atomic number = 23 is of vanadium. The hydride is VH. It is interstitial or metallic. Atomic number =44 is of ruthenium, its hydrid
Similar Questions for you
H2S has minimum boiling point.
PbS + 4H2O2->PbSO4 + 4H2O.
Volume strength of H2O2 = Molarity * 11.2
HF molecules are associated with strong intermolecular hydrogen bonding hence its boiling point is the highest
Compound Melting Point (K)
HF 190
HCl
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering