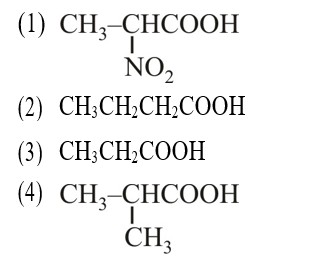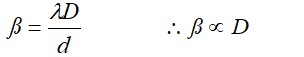A 500g tooth-paste sample has 0.2g fluoride concentration. What is the concentration of fluoride in terms of ppm level ?
A 500g tooth-paste sample has 0.2g fluoride concentration. What is the concentration of fluoride in terms of ppm level ?
As 500g of tooth-paste has = 0.2g of F?
So, 10 g of toothpaste has = (0.2g * 10? g) / 500g F = 400g of F
Hence, concentration in ppm is 400.
Similar Questions for you
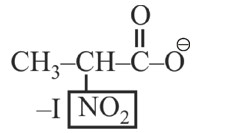
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering