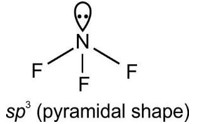
A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The shape of this molecule is:
A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The shape of this molecule is:
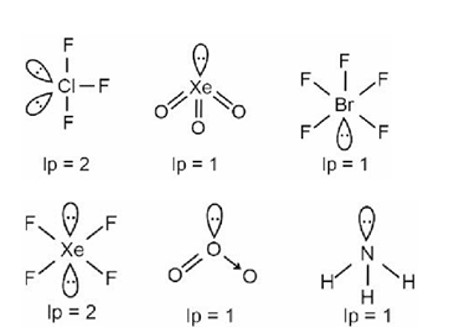
A central atom having two lone pairs and three bond pairs reflects sp³d hybridization and a corresponding T-shaped geometry.
Similar Questions for you
PF5, PCl5, PBr5, Fe (CO)5 Þ Trigonal bipyramidal
BrF5 Þ Square pyramidal
[PtCl4]2– Þ Square planar
SF6 Þ Octahedral
During the electrolysis of dilute H2SO4
In the solid form of dihedral angle is equal to 90.2°.
Total number of electron in Ti = 22
Total number of electron in Ti? = 22 – 4 = 18 So EAN value of Ti = 18 + 12 + 4 = 34
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Chemical Bonding and Molecular Structure 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering