Ammonolysis of Alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction is:
Ammonolysis of Alkyl halides followed by the treatment with NaOH solution can be used to prepare primary, secondary and tertiary amines. The purpose of NaOH in the reaction is:
In the ammonolysis reaction, HCl is produced as a byproduct. To neutralize this acidic impurity, the mixture is treated with NaOH.
Similar Questions for you
In Amines, the nitrogen atom bonds with alkyl or aryl groups replacing hydrogen, whereas in amides, the nitrogen atom bonds directly with the carbonyl group (-CO-).
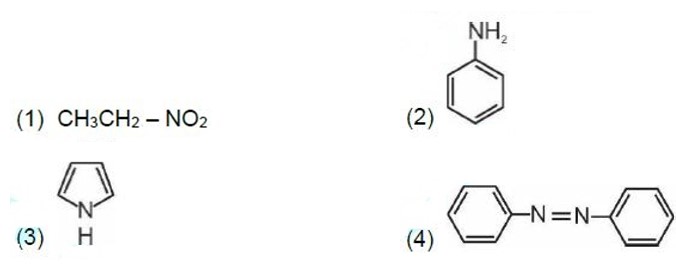
Kjeldahl's method is not applicable to compounds containing nitrogen in nitro and azo groups and nitrogen present in the ring.
Correct order of basic strength in aqueous medium is
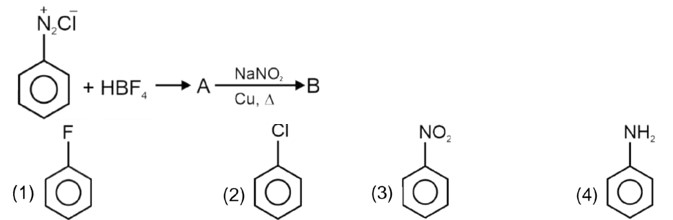
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering