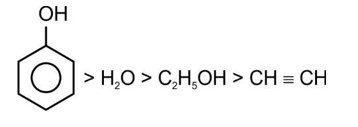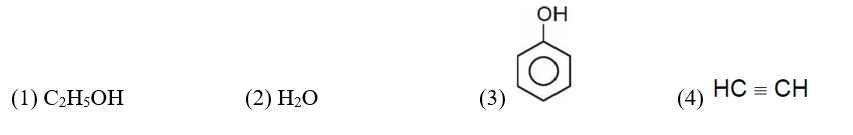Arrange the following compounds in increasing order of boiling point. Propan- 1-ol. butan-l-ol. butan-2-ol. pentan- 1-ol
I. Propan- 1-ol. butan-2-ol. butan- 1-ol. pentan- 1-ol
II. Propan- 1 -ol. butan- 1 -ol. butan-2-ol. pentan- 1 -ol
III. Pentan-l-ol. butan-2-ol. butan- 1 -ol. propan- 1-ol
IV. Pentan-l-ol. butan- 1-ol. butan-2-ol. propan- 1-ol
Arrange the following compounds in increasing order of boiling point. Propan- 1-ol. butan-l-ol. butan-2-ol. pentan- 1-ol
I. Propan- 1-ol. butan-2-ol. butan- 1-ol. pentan- 1-ol
II. Propan- 1 -ol. butan- 1 -ol. butan-2-ol. pentan- 1 -ol
III. Pentan-l-ol. butan-2-ol. butan- 1 -ol. propan- 1-ol
IV. Pentan-l-ol. butan- 1-ol. butan-2-ol. propan- 1-ol
This is a multiple choice answer as classified in NCERT Exemplar
(I) Propan- 1-ol. butan-2-ol. butan- 1-ol. pentan- 1-ol
The compounds Propan-l-ol, butan-2-ol, butan-1-ol, pentan-1-ol belong to the alcohol family. The structure of the given alcohols are as shown below:
With increase in the molecular
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering