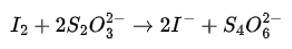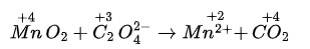Balance the following equations by the oxidation number method
(i) Fe2+ + H+ + Cr22–O7 → Cr3+ + Fe3+ + H2O
(ii) I2 + NO3– → NO2 + IO3
–
(iii) I 2 + S2O32– → I– + S4O62–
(iv) MnO2 + C2 O42– → Mn2+ + CO2
Balance the following equations by the oxidation number method
(i) Fe2+ + H+ + Cr22–O7 → Cr3+ + Fe3+ + H2O
(ii) I2 + NO3– → NO2 + IO3 –
(iii) I 2 + S2O32– → I– + S4O62–
(iv) MnO2 + C2 O42– → Mn2+ + CO2
-
1 Answer
-
This is a Short answer type question as classified in NCERT Exemplar
(i) We can balance the given equation by oxidation number method-
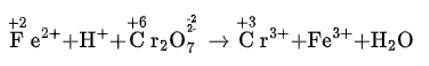
(a)Balance the increase and decrease in O.N.
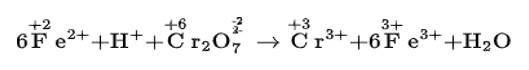
(b) Balancing H and O atoms by adding H+ and H2O molecules
(ii) We can balance the given equation by oxidation number method-
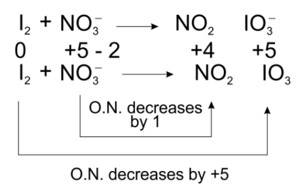
Total decrease in O.N. = 1
To equilize O.N. multiply NO3-, by 10
I2 + 10 NO3-? 10NO2 + IO3-
Balancing atoms other than O and H
I2 + 10 NO3-? 10NO2 + 2 IO3-
Balancing O and H
I2 + 10 NO3- + 8H+? 10NO2 + 2 IO3- + 4H2O
(iii) We can balance the given equation by oxidation number method
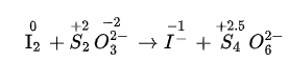
Total increase in O.N. =
...more
Similar Questions for you
Kindly go through the solution
(c) Li
Kindly go through the solution
(c) Al
Kindly go through the solution
(d) +6
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers