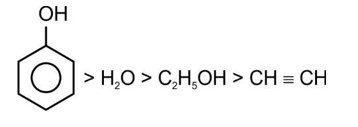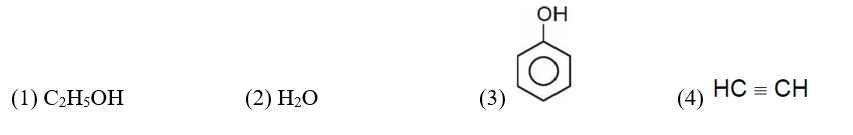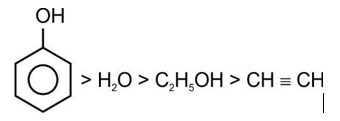Consider the following molecules and statements related to them:
Molecule (P) is p-nitrophenol.
Molecule (Q) is o-nitrophenol.
(a) (Q) is more volatile than (P)
(b) (Q) has higher boiling point than (P)
(c) (P) dissolves more readily than (Q) in water
(d) pKa value of (P) is higher than (Q)
Identify the correct option from below:
Consider the following molecules and statements related to them:
Molecule (P) is p-nitrophenol.
Molecule (Q) is o-nitrophenol.
(a) (Q) is more volatile than (P)
(b) (Q) has higher boiling point than (P)
(c) (P) dissolves more readily than (Q) in water
(d) pKa value of (P) is higher than (Q)
Identify the correct option from below:
Due to Intramolecular hydrogen bonding, O-nitrophenol fails to spread its surface, so that its b.p is less than para-nitrophenol.
Similar Questions for you
Rainbow is formed due to internal reflection and dispersion.
Correct order of acidic strength
Correct order of acidic strength
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering