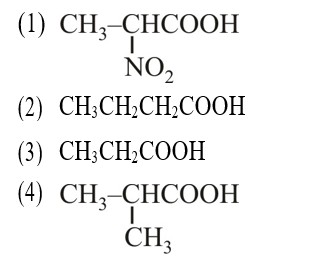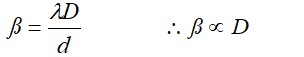Dinitrogen and dioxygen are main constituents of air, but these do not react with each other to form oxides of nitrogen because _________.
(i) The reaction is endothermic and requires very high temperatures.
(ii) The reaction can be initiated only in presence of a catalyst.
(iii) Oxides of nitrogen are unstable.
(iv) N2 and O2 are unreactive.
Dinitrogen and dioxygen are main constituents of air, but these do not react with each other to form oxides of nitrogen because _________.
(i) The reaction is endothermic and requires very high temperatures.
(ii) The reaction can be initiated only in presence of a catalyst.
(iii) Oxides of nitrogen are unstable.
(iv) N2 and O2 are unreactive.
-
1 Answer
-
This is a Multiple choice Questions as classified in NCERT Exemplar
Option (i) the reaction is endothermic and requires very high temperature is correct since dinitrogen and dioxygen are the main constituents of air. These gases do not react with each other at a normal temperature. The dissociation energy of N2is very high due to the presence of triple bond and it is very stable.
Similar Questions for you
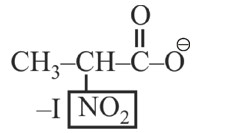
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers