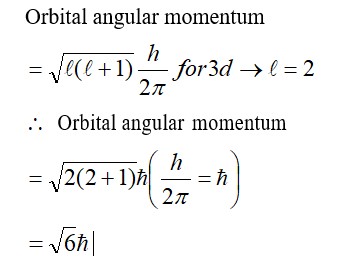
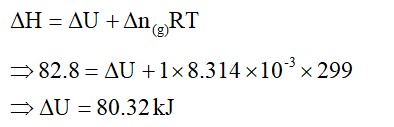Extensive properties depend on the quantity of matter but intensive properties do not. Explain whether the following properties are extensive or intensive. Mass, internal energy, pressure, heat capacity, molar heat capacity, density, mole fraction, specific heat, temperature and molarity.
Extensive properties depend on the quantity of matter but intensive properties do not. Explain whether the following properties are extensive or intensive. Mass, internal energy, pressure, heat capacity, molar heat capacity, density, mole fraction, specific heat, temperature and molarity.
-
1 Answer
-
This is a Long Answer Type Questions as classified in NCERT Exemplar
A contrast is drawn in thermodynamics between extensive and intense qualities. An extensive property is one whose value is proportional to the amount or size of matter in the system. Extensive properties include mass, volume, internal energy, enthalpy, and heat capacity, to name a few.
Properties that are independent of the amount or size of matter present are known.
As though they were intensive properties Temperature, density, and pressure, for example, are intense properties. A molar property? m, is the value of an extensive property of the system for 1 mol of the sub
...more
Similar Questions for you
Kindly go through the solution
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers