For an ideal gas, the work of reversible expansion under isothermal condition can be calculated by using the expression w= -nRTln
A sample containing 1.0 mol of an ideal gas is expanded isothermally and reversibly to ten times of its original volume, in two separate experiments. The expansion is carried out at 300 K and at 600 K respectively. Choose the correct option.
(i) Work done at 600 K is 20 times the work done at 300 K.
(ii) Work done at 300 K is twice the work done at 600 K.
(iii) Work done at 600 K is twice the work done at 300 K.
(iv) ∆U = 0 in both cases.
For an ideal gas, the work of reversible expansion under isothermal condition can be calculated by using the expression w= -nRTln
A sample containing 1.0 mol of an ideal gas is expanded isothermally and reversibly to ten times of its original volume, in two separate experiments. The expansion is carried out at 300 K and at 600 K respectively. Choose the correct option.
(i) Work done at 600 K is 20 times the work done at 300 K.
(ii) Work done at 300 K is twice the work done at 600 K.
(iii) Work done at 600 K is twice the work done at 300 K.
(iv) ∆U = 0 in both cases.
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iv)
For
isothermal reversible change
q= -w = nRTln =2.303nRTlog
= = =2
For
isothermal expansion of ideal gases, ? U = 0
Since,
temperature is constant this means there is no change in internal energy.
Similar Questions for you
Kindly go through the solution
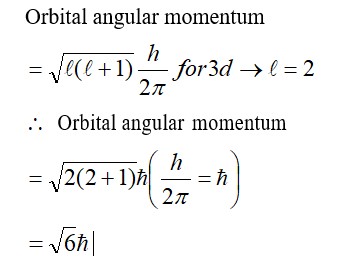
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Six 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering