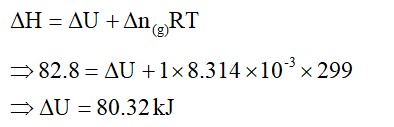ΔG is net energy available to do useful work and is thus a measure of “free energy”. Show mathematically that ΔG is a measure of free energy. Find the unit of ΔG. If a reaction has positive enthalpy change and positive entropy change, under what condition will the reaction be spontaneous?
ΔG is net energy available to do useful work and is thus a measure of “free energy”. Show mathematically that ΔG is a measure of free energy. Find the unit of ΔG. If a reaction has positive enthalpy change and positive entropy change, under what condition will the reaction be spontaneous?
-
1 Answer
-
This is a Long Answer Type Questions as classified in NCERT Exemplar
We know,
ΔStotal = ΔSsys + ΔSsurr
When a system is in thermal equilibrium with its surroundings, the surroundings' temperature is the same as the system's. Furthermore, a rise in the enthalpy of the surroundings equals a decrease in the system's enthalpy. As a result of the entropy shift in the environment,
ΔSsurr = ΔHsurr/T = -ΔHsys/T
ΔStotal = ΔSsys = (-ΔHsys/T)
Rearranging the above equation:
ΔStotal = TΔSsys - ΔHsys
For spontaneous process,
ΔStotal > 0, so
TΔSsys - ΔHsys> 0
&rA
...more
Similar Questions for you
Kindly go through the solution
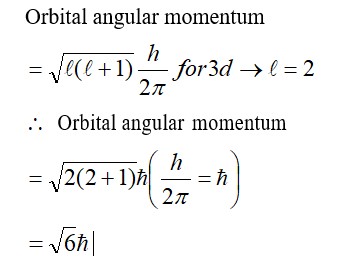
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers