Give reasons for the following :
(i) Covalent bonds are directional bonds while ionic bonds are nondirectional.
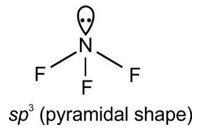
(ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(iii) Ethyne molecule is linear.
Give reasons for the following :
(i) Covalent bonds are directional bonds while ionic bonds are nondirectional.
(ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(iii) Ethyne molecule is linear.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Ans: (i) Due to the electrostatic forces between the two opposite charges, ionic bonds are non-directional. The bonding direction does not matter as the electrostatic field of an ion is non-directional. Whereas, the formation of c
Similar Questions for you
He2 has zero bond order hence it does not exist.
The three fundamental laws of chemistry are - Law of Definite Proportions, Law of Conservation of Mass, and Law of Multiple Proportions.
The three types of chemical bonds are - ionic, metallic and covalent bonds. When the electrons transfer between the atoms, they form the Ionic bonds by producing charged ions that are attracted to each other. When atoms share electrons, covalent bonds are created. When metal atoms share a sea of del
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Four 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering