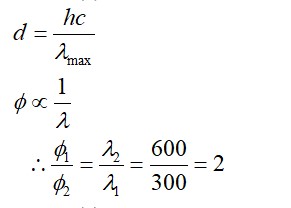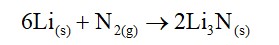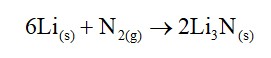Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Lithium salts are hydrated.
Reason (R) : Lithium has higher polarizing power than other alkali metal group members.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Lithium salts are hydrated.
Reason (R) : Lithium has higher polarizing power than other alkali metal group members.
In the light of the above statements, choose the most appropriate answer from the options given below:
Lithium salts are extensively hydrated due to high hydration enthalpy of Li+
(order of polarizing power)
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering