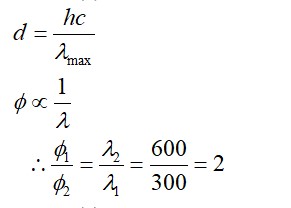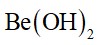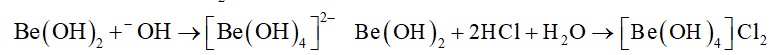Given below are two statements:
Statement I : Both CaCl2.6H2O and MgCl2.8H2O undergo dehydration on heating.
Statement II : BeO is amphoteric whereas the oxides of other elements in the same
group are acidic.
In the light of the above statements, choose the correct answer from the options given below:
Given below are two statements:
Statement I : Both CaCl2.6H2O and MgCl2.8H2O undergo dehydration on heating.
Statement II : BeO is amphoteric whereas the oxides of other elements in the same
group are acidic.
In the light of the above statements, choose the correct answer from the options given below:
Hydrated halides of group 2 from Ca onwards undergo dehydration on heating. But the hydrated halide of Mg, i.e., MgCl? ·8H? O, does not undergo dehydration on heating but undergoes hydrolysis.
BeO is amphoteric, while other oxides of group 2 are basic in nature.
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering