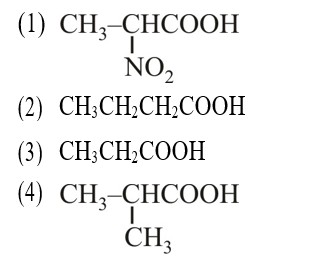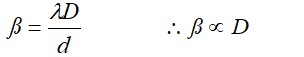Given below are two statements:
Statement I: Chlorofluoro carbons breakdown by radiation in the visible energy region and release chlorine gas in the atmosphere which then reacts with stratospheric ozone.
Statement II: Atmospheric ozone reacts with nitric oxide to give nitrogen and oxygen gases, which add to the atmosphere.
For the above statements choose the correct answer from the options given below:
Given below are two statements:
Statement I: Chlorofluoro carbons breakdown by radiation in the visible energy region and release chlorine gas in the atmosphere which then reacts with stratospheric ozone.
Statement II: Atmospheric ozone reacts with nitric oxide to give nitrogen and oxygen gases, which add to the atmosphere.
For the above statements choose the correct answer from the options given below:
Option 1 -
Statement I is incorrect but Statement II is true
Option 2 -
Both Statement I and II are correct
Option 3 -
Both Statement I and II are false
Option 4 -
Statement I is correct but Statement II is false
-
1 Answer
-
Correct Option - 1
Detailed Solution:CFC breakdown by visible light to give Cl radical which react with stratospheric ozone.
CFC, CF? Cl? - (hv)-> Cl• + •CF? Cl
Cl• (g) + O? → ClO• + O?
ClO• + O → Cl• + O?
Atmospheric ozone reacts with NO to give NO? and O?
O? + NO → NO? + O?
Similar Questions for you
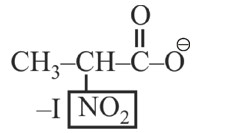
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers