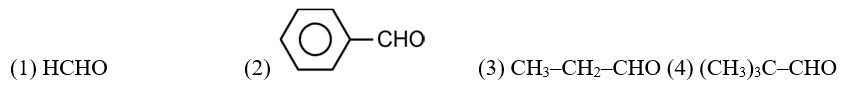
Given below are two statements:
Statement I:
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole-dipole interactions.
Statement II:
The boiling points of aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements:
Statement I:
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole-dipole interactions.
Statement II:
The boiling points of aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the above statements, choose the most appropriate answer from the options given below:
As compared to hydrocarbons of similar mass aldehydes and ketones will have greater dipole-dipole interactions.
Similar Questions for you
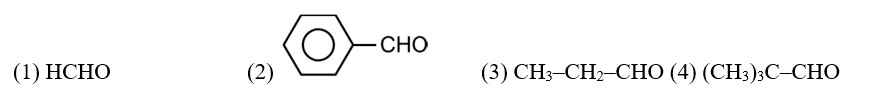
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Acetaldehyde (CH3CHO) gives positive lodoform test and positive Fehling's solution test
CH3—CH2—CHO does not undergo Cannizzaro reaction because it has α-hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering