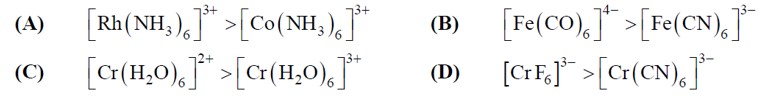How are metal-carbon bonds (M-C) formed in metal carbonyls?
How are metal-carbon bonds (M-C) formed in metal carbonyls?
The metal-carbon bonds (M-C) in metal carbonyls are due to the synergic interaction between the metal and carbonyl group. There are two types of bonding between metal and carbonyl group.
? -bond in Metal Carbonyls: The Carbonyl (CO) ligand donates a lone pair of electrons to the metal center to fill
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
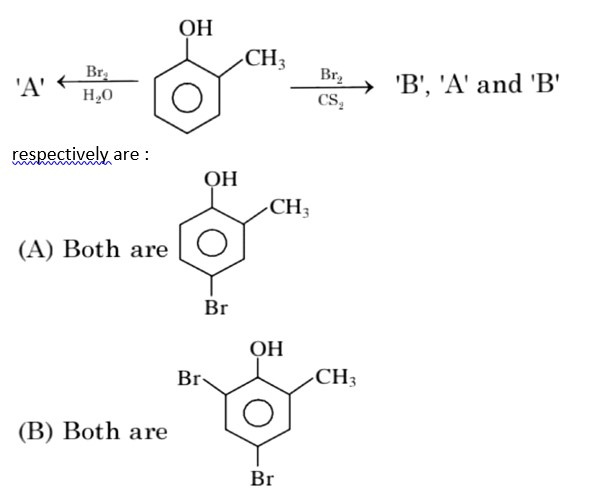
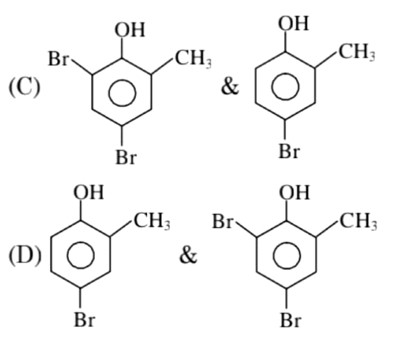
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Coordination Compounds 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering