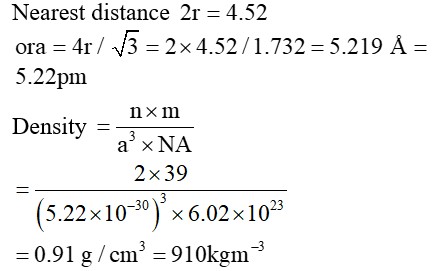
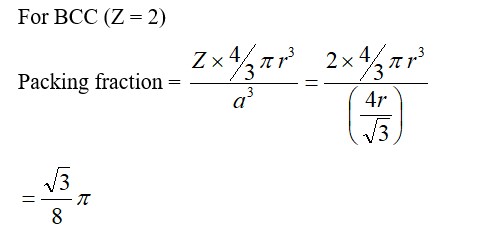Potassium (K) has a bcc structure with nearest neighbor distance 4.52 A0 Its atomic weight is 39. Its density will be
Potassium (K) has a bcc structure with nearest neighbor distance 4.52 A0 Its atomic weight is 39. Its density will be
Option 1 - <p>910 Kgm<sup>-3</sup></p>
Option 2 - <p>804Kgm<sup>-3</sup></p>
Option 3 - <p> 454Kgm<sup>-3</sup></p>
Option 4 - <p>852 Kgm<sup>-3</sup></p>
3 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
A
Answered by
5 months ago
Correct Option - 1
Detailed Solution:
Please find the attached solution:
Similar Questions for you
ΔG° = –RT * 2.303 log K
–nFE° = +RT * 2.303 log K
2 * 96500 * 0.295 = 8.314 * 298 * 2.303 log10 K
10 = log10 K = 1010
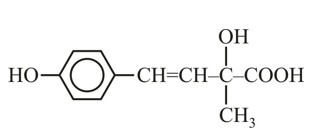
It has chiral centre and differently di substituted double bonded carbon atoms.
For FCC lattice
Packing efficiency = 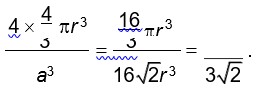
CsCl has BCC structure in which Cl– is present at corners of cube and Cs+ at body centre
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering