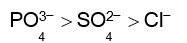If the initial pressure of a gas is 0.03 atm, the mass of the gas adsorbed per gram of the adsorbent is____________* 10-2g.
If the initial pressure of a gas is 0.03 atm, the mass of the gas adsorbed per gram of the adsorbent is____________* 10-2g.
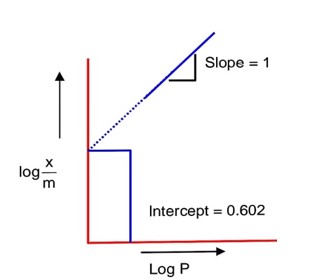
Using, Freundlich adsorption isotherm ;
………………. (i)
Comparing with y = mx + C
Slope =
Intercept, log k = 0.602
log k = log 4
k = 4
from equation (i)
= 0.12
= 12 * 10-2
So, 12 * 10-2 g of gas is adsorbed per gram of adsorbent,
Similar Questions for you
The process of settling of colloidal particles is
In physisorption multimolecular layers are formed on solid surface.
Emulsion is a colloidal solution of liquid in liquid.
Haemoglobin is a positive colloid. Hence greater is the charge of anion, more effective will be the coagulation of haemoglobin.
Therefore,
Correct order of coagulating power is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Five 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering