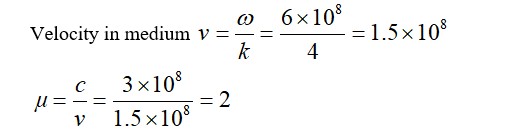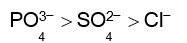In physisorption adsorbent does not show specificity for any particular gas because
(i) Involved van der Waals forces are universal.
(ii) Gases involved behave like ideal gases.
(iii) Enthalpy of adsorption is low.
(iv) It is a reversible process.
In physisorption adsorbent does not show specificity for any particular gas because
(i) Involved van der Waals forces are universal.
(ii) Gases involved behave like ideal gases.
(iii) Enthalpy of adsorption is low.
(iv) It is a reversible process.
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (i)
Physisorption adsorbent does not show specificity for any particular gas because it is involved van there Waals forces are universal, hence the correct option is (i).
Similar Questions for you
The process of settling of colloidal particles is
In physisorption multimolecular layers are formed on solid surface.
Emulsion is a colloidal solution of liquid in liquid.
Haemoglobin is a positive colloid. Hence greater is the charge of anion, more effective will be the coagulation of haemoglobin.
Therefore,
Correct order of coagulating power is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Five 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering