In the graph showing Maxwell Boltzmann distribution of energy, ___________.
A. Area under the curve must not change with increase in temperature.
B. Area under the curve increases with increase in temperature.
C. Area under the curve decreases with increase in temperature.
D. With increase in temperature curve broadens and shifts to the right-hand side.
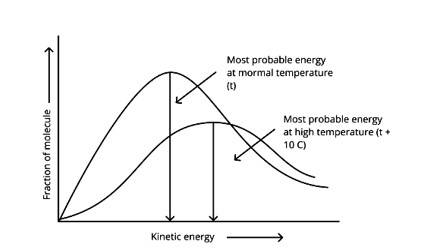
In the graph showing Maxwell Boltzmann distribution of energy, ___________.
A. Area under the curve must not change with increase in temperature.
B. Area under the curve increases with increase in temperature.
C. Area under the curve decreases with increase in temperature.
D. With increase in temperature curve broadens and shifts to the right-hand side.

-
1 Answer
-
This is a Multiple Choice Questions as classified in NCERT Exemplar
Correct options: A and D
According to the graph, the area under the cure must not vary as the temperature rises.T2 > T1
The energy level of T2 is higher than T1.
Similar Questions for you
Kindly go through the solution
Ea = 216.164kJ/mol 216
Reaction rate is used to measure how fast or slow reactions occur per unit time. The rate constant is a proportionality factor that remains constant for every reaction.
Yes, in elementary reactions, order and molecularity can be the same, but this is not always the case because order is an experimental quantity, and molecularity is a theoretical concept.
Reaction Kinetics, also known as chemical kinetics, is the study of the rate of chemical reaction and the factors affecting the reaction rate, such as temperature, concentration, and catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
