Match the statements given in Column I and Column II

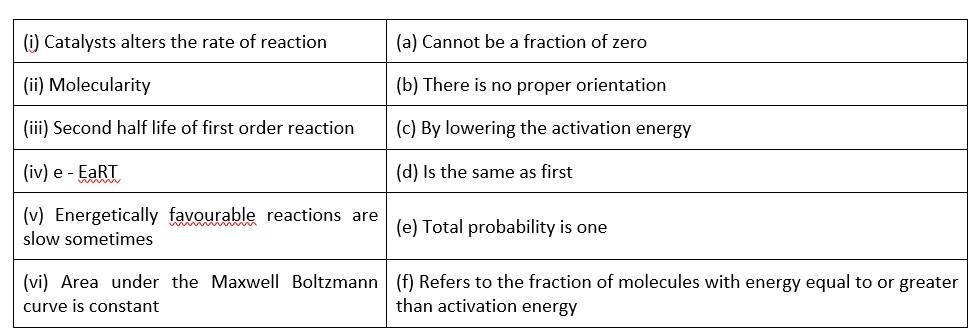
Match the statements given in Column I and Column II


-
1 Answer
-
This is a Matching Type Question as classified in NCERT Exemplar
(i)- (c) ; (ii)- (a) ; (iii)- (d); (iv)- (f); (v)- (b); (vi)- (b)
A catalyst can influence the rate of a process by lowering the activation energy
When it comes to molecularity, there can't be a fraction or a zero.
The second half life of a first order reaction is the same as the first.
Activation energy refers to the percentage of molecules with an energy equal to or greater than activation energy.
Correct orientation is not always present when it comes to energetically favourable activities.
Because the area under the Maxwell Boltzmann curve is constant, the total probability
...more
Similar Questions for you
Kindly go through the solution
Ea = 216.164kJ/mol 216
Reaction rate is used to measure how fast or slow reactions occur per unit time. The rate constant is a proportionality factor that remains constant for every reaction.
Yes, in elementary reactions, order and molecularity can be the same, but this is not always the case because order is an experimental quantity, and molecularity is a theoretical concept.
Reaction Kinetics, also known as chemical kinetics, is the study of the rate of chemical reaction and the factors affecting the reaction rate, such as temperature, concentration, and catalyst.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
