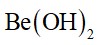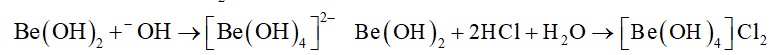Name an element from Group 2 which forms an amphoteric oxide and a water soluble sulphate
Name an element from Group 2 which forms an amphoteric oxide and a water soluble sulphate
2 Views|Posted 8 months ago
Asked by Shiksha User
1 Answer
P
Answered by
8 months ago
This is a short answer type question as classified in NCERT Exemplar
The element from group 2 is beryllium. Be (OH)2 is amphoteric. It reacts with both acids and bases. It reacts with acid to form beryllium chloride and it reacts with base to form beryllate ion which is soluble in sodium hydroxide. T
Similar Questions for you
Li+ has the highest hydration enthalpy.
Hence it is most hydrated
Therefore, Correct order of hydrated radii is Cs+ < Rb+ < K+ < Na+ < Li+
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Ten 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering