Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
Noble gases are named because of their inertness towards reactivity. Identify an incorrect statement about them.
Noble gases have weak dispersion forces so their melting and boiling point are very low.
Similar Questions for you
In (Ph)2C = CHCN3, no spatial variation is possible
It will not show geometrical isomerism.
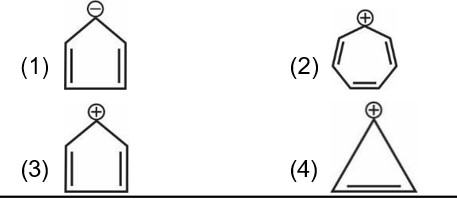
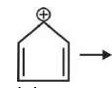
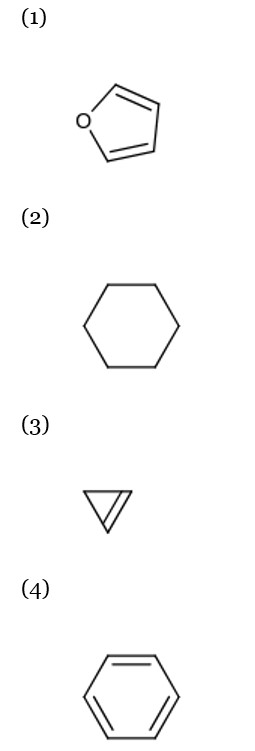
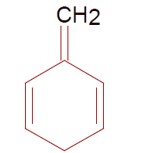
Cyclohepta-1, 3, 5-triene is not aromatic as one carbon is saturated (sp³).
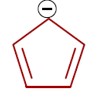
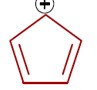
In an aromatic compound, overall delocalization is processed by the electrons according to Hückel's rule. For the compound in question, the total number of delocalized electrons is 6.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering