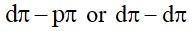On balancing the given redox reaction, aCr₂O₇²⁻(aq) + bSO₃²⁻(aq) + cH⁺(aq) → 2aCr³⁺(aq) + bSO₄²⁻(aq) + c/2H₂O(l) the coefficients a, b and c are found to be, respectively:
On balancing the given redox reaction, aCr₂O₇²⁻(aq) + bSO₃²⁻(aq) + cH⁺(aq) → 2aCr³⁺(aq) + bSO₄²⁻(aq) + c/2H₂O(l) the coefficients a, b and c are found to be, respectively:
Reduction half reaction.
Cr? O? ²? + 14H? + 6e? → 2Cr? ³ + 7H? O
Oxidation half reaction
SO? ²? + H? O → SO? ²? + 2e? ] * 3
Oxygen is balanced by adding water and hydrogen is balanced by adding H? and the charge is balanced by electrons.
Add ( eq. (i) + (3 * eq. (ii) )
Cr? O? ²? + 3SO? ²? + 8H? → 2Cr? ³ +
Similar Questions for you
Na+ C + N + S ®NaSCN
Fe3+ + SCN–
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Ellingham diagram explains the feasibility of reduction process not the kinetics of process.
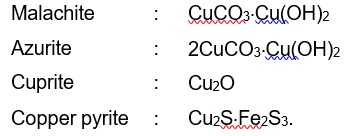
Malachite : CuCO3.Cu (OH)2
Azurite : 2CuCO3.Cu (OH)2
Cuprite : Cu2O
Copper pyrite : Cu2S.Fe2S3.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering